Ion Production Environments
Figure: NH2+ and HCO+ Signal Strength. Signal
strength of NH2+ (squares) and HCO+ (triangles) function of
magnetic field.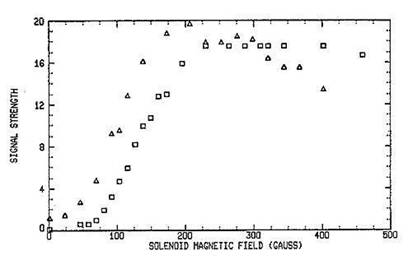
The basis of our experimental work in this field was the development of a technique for ion
spectroscopy that produces signals two orders of magnitude larger than previous techniques [1]. This is especially important because prior to this
development, microwave ion spectroscopy was reputed to be extremely difficult and time consuming because
of the long searches for weak lines. The basis of the method is the extension of the ion rich negative
glow region of an anomalous glow discharge by means of an axial magnetic field. In addition to the very
large gains in signal strength (~x100), the enhancement is also ion specific, thus providing a powerful
discriminant.
Figure: NH2+ and HCO+ Signal Strength. Signal
strength of NH2+ (squares) and HCO+ (triangles) function of
magnetic field.
In [1].it was pointed out that the magnetically lengthened negative glow cell is functionally equivalent to an electron gun injecting magnetically confined electrons into a electric field free region. Based on this equivalence a new cell has been developed.
Figure: Magnetic Enhancement. Cell for the study of molecular ions Figure: Cell for Molecular Ion Study.
based on the magnetic enhancement of the negative glow region of a
discharge.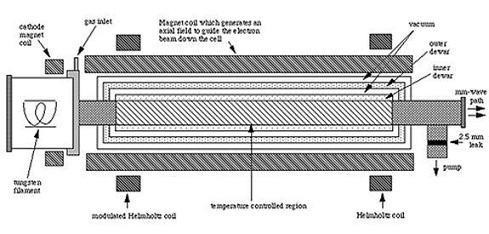
In the cell shown in Figure: Cell for Molecular Ion Study the electrons are produced by a thermonic cathode and guided through a metallic tube which can be cooled either by liquid helium or liquid nitrogen. Because the requirements for sustaining an anomolous glow discharge place severe constraints on pressure, gas mixture, voltage, and current, this new cell provides significantly greater flexibility for the optimization of the production of ions. Additionally, because the parameters of the systems are much better determined, it is possible to model the ion production. This makes possible more predictive ion production schemes as well as the quantitative measurement of the physical and chemical parameters involved.
Figure: Pulsed E-beam THz Probe. THz probe of a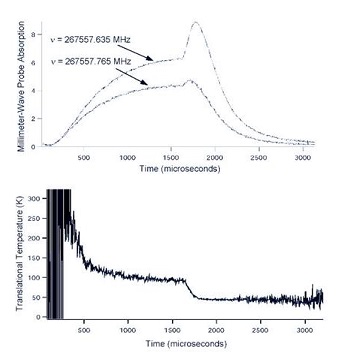
pulsed e-beam induced ion-molecule reaction in a low
temperature H2 - CO mixture, showing (upper) the
initial formation of the HCO+ (the initial rise in probe
absorption), the rotational and translational cooling
of the average temperature after the end of the e-
beam pulse (the sharp rise at 1.8 msec), and the
eventual recombination of the HCO+ and the slow
electrons. The lower portion shows the translational
temperature calculated from the Doppler lineshape
information provided by the two probes shown in
the upper figure which probe different parts of the
Gaussian lineshape.
In addition to the spectroscopic study of energy levels, the THz has been used to probe both discharge [7]. Figure: Pulsed E-beam THz Probe shows a recent result which combines many of these experimental technique to establish an experimentally well characterized environment for the study of inelastic collision of molecular ions at very low temperatures.
More specifically, this figure shows an example of the results of a THz study of the chemistry and
physics which results from a pulsed flux of electrons being focused through a mixture of H2
and CO at ~ 50 K to form HCO+. This experimental system is similar to that shown in
Figure: Magnetic Enhancement. Cell for the study of molecular ions
based on the magnetic enhancement of the negative glow region of a
discharge.
References
- The Production of Large Concentrations of Molecular Ions in the Lengthened Negative Glow Region of a Discharge J. Chem. Phys. 78, 2312-2316 (1983). Google Scholar
- Millimeter-Wave Spectroscopy in Electric Discharges. Rare Molecules Show Themselves Only If You Look In The Other Direction J. Mol. Spectrosc. 168, 215-226 (1994). Google Scholar
- Laboratory Detection of the 110 - 111 Submillimeter Wave Transition of the H2D+ Ion J. Chem. Phys. 81, 2514 (1984). Google Scholar
- Laboratory Measurement of the 110 - 111 Submillimeter Line of H2D+ Astron. Astrophys. 137, L15-L16 (1984). Google Scholar
- Laboratory Measurement of the Pure Rotational Spectrum of Vibrationally Excited HCO+ (v2 = 1) by Far-Infrared Laser Sideband Spectroscopy Astrophys. J. 316, L45-L48 (1987). Google Scholar
- Double-Modulation Submillimeter-Wave Spectroscopy of HOC+ in the nu2 Excited Vibrational State J. Mol. Spectrosc. 203, 140-144 (2000). Google Scholar
- Dynamics of the HCN Discharge Laser Appl. Phys. Lett. 46, 631-633 (1985). Google Scholar
- Collisional Energy Transfer in Optically Pumped Far-Infrared Lasers IEEE J. Quantum Electron. 23, 2069-2077 (1987). Google Scholar
- Rotational Energy Transfer in Small Polyatomic Molecules Adv. At., Mol., Opt. Phys. 35, 331-400 (1995). Google Scholar
- Millimeter-Wave Time-Resolved Studies of HCO+ - H2 Inelastic Collisions Spectrochim. Acta, Part A 57, 705-716 (2001). Google Scholar