Gases, Liquids, and Solids
Figure: Rotational Interaction Strength. Rotational interaction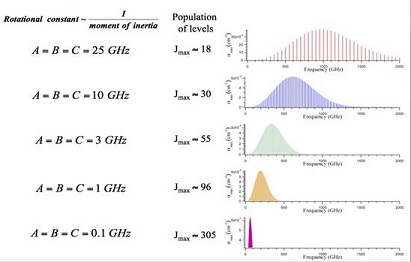
strength in the THz spectral region as a function of molecular size (i.e.
the molecular moments of inertia) and frequency.
Figure: Rotational Interaction Strength shows the strength of interactions as a function of frequency and molecular mass [1]. These absorptions result from the interaction of the rotation of the molecule with the radiation and exist only in gases. This figure shows that the strengths of the interactions increase as $ν^2 - ν^3$. As a result, THz interactions are 103 - 106 times more intense than interactions in the conventional Microwave (MW) region. Beyond a mass dependent peak in the THz, the interaction strength falls exponentially towards the infrared. This very sharp peak in the THz is one of the most important features of the spectral region and is closely related to many of the current and potential applications.
Figure: Atmospheric Propagation. Atmospheric
propagation as a function of altitude in the THz spe-
ctral region. Note the rapid change with altitude, both
due to the narrowing of the lines and the drying of
the atmosphere with altitude.
Figure: Atmospheric Propagation. Atmospheric
propagation as a function of altitude in the THz spe-
ctral region. Note the rapid change with altitude, both
due to the narrowing of the lines and the drying of
the atmosphere with altitude.
All of the interactions discussed above are based on the interaction of molecular rotation with THz radiation. In liquids and solids there is no rotation and consequently no rotational spectra. However, for large molecules collective motions are possible which result in energy level spacings that correspond to THz frequencies. Solids and liquids are strongly interacting and as a result resonances are much broader, ordinarily leading to 'continua' spectra in the THz. However, evidence of specific features in biological substances has been reported. Although speculative, it would appear to us that such spectra are most likely at relatively high THz frequencies.
References
- FASSST: A new Gas-Phase Analytical Tool Anal. Chem. 70, 719A-727A (1998). Google Scholar